| Case Name |
Silane Gas Explosion at Osaka University |
| Pictograph |
|
| Date |
October 2, 1991 |
| Place |
Osaka, Japan |
| Location |
School of Engineering Science, Osaka University |
| Overview |
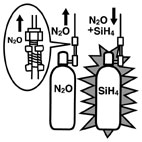
An explosion occurred at the School of Engineering Science, Osaka University, during a student experiment with a CVD (Chemical Vapor Deposition) system. The Silane container (Figure 1), which supplied gas to the CVD system, blew up. Two students were killed and five others received minor injuries. The accident was caused by a reverse flow of Nitrous Oxide due to a degraded O-ring seal used in a check valve (Figure 2), and the mixture of Silane and Nitrous Oxide exploded in the container. The explosion ignited the city gas and organochlorine solvent in the facility and started a fire. |
| Incident |
During student experiments at the School of Engineering Science, Osaka University with a plasma CVD system, a Silane container that supplied gas to the CVD system suddenly exploded. Two students were killed and five others received minor injuries by the blast and flying debris from the explosion. |
| Sequence |
Around 4pm on October 2, 1991, at the School of Engineering Science, Osaka University, a student closed a valve (DV1 in Figure 1) of a plasma CVD system. The closure triggered a sudden explosion in the Silane container that supplied gas to the CVD system. The Silane container bursted, and the blast forced everything including the test equipment and people to the walls. The explosion and flying debris from it killed two students and injured five others. The explosion also ignited the city gas and organochlorine solvents to start a fire. |
| Cause |
What exploded in the Silane container was mixed gas of Silane (SiH4) and Nitrous Oxide (N2O). The O-ring within check valve (CV3) in Figure 2 had been degraded by Nitrous Oxide, and the check valve (i.e., non-return valve) had lost its function. As a result, a reverse flow of Nitrous Oxide through the check valve (CV3) flew into the Silane container via the purge lines. The purge lines remove noxious fumes and combustible gas inside the pipes by introducing inert gas such as Nitrogen after each system operation. When the student closed the valve (DV1) the mixed gas in purge lines compressed, generated heat and ignited. The flame then traveled back to the container through the piping and exploded the container. The pressure inside the container at the time of explosion was estimated at 2,000 to 3,000kgf/cm2. The safety valves for preventing heat meltdown of the container had no time to activate. |
| Response |
Two months after the incident, High-pressure Gas Control Law was revised to mandate reporting the use of specific high-pressure gas such as Silane, regardless of the amount. The revision was posted to jurisdictional prefecture and city governments. Each university voluntarily started inspecting applicable gas lines. |
| Countermeasures |
(1) To prevent the O-ring degradation by Nitrous Oxide, the check valve (CV3) and the ball valve (BV3) were relocated. The original location of the check valve close to a gas cylinder kept the O-ring exposed to Nitrous Oxide. The pressure of Nitrous Oxide (typically 50 atmospheres) is higher than the pressure of Nitrogen (usually a few atmospheres). A new ball valve with metal contact was placed next to the gas cylinder.
(2) Separate purge lines were placed for each gas type. Shared pipes have advantage in easy operation and lower system cost, however, could generate dangerous gas mixture like in this case. |
| Knowledge Comment |
(1) Silane gas is dangerous. It can explode when its density exceeds 1%, and is flammable when mixed with Nitrous Oxide. The mixture usually does not explode at room temperature, but if it is ignited the power of explosion is large.
(2) Degradation of material may lead to an accident. Systems should use material that is hard to degrade in the planned environment, or otherwise, they should be designed with inherent safety to prevent damage even when the material degrades.
(3) Efforts to improve working property or reduce cost can reduce the margin of safety.
(4) If fatal accidents are possible, designing inherent safety into systems is absolutely essential. The original layout in Figure 1 is dangerous, for example, if an earthquake happens during an experiment, piping can get slacked to mix gases, and any spark can readily cause an explosion like this case.
(5) Gas-leak sensors are not versatile. It appears that they thought the system was safe enough with the gas-leak sensor (indicated in the center of Figure 1). |
| Background |
Technopolis Initiative (Law for Accelerating Regional Development Based upon High-Technology Industrial Complexes: Concept to develop industrial towns, which organically bound academic research functions and living functions, in order to introduce and develop high-tech industry to local regions) driven by the Ministry of International Trade and Industry (now the Ministry of Economy, Trade and Industry) went into effect in March 1983. The initiative triggered rapid introduction of foreign technology with foci on semiconductor technology and mechatronics. The semiconductor technology involves various chemical materials including Silane. Many accidents were caused by the spontaneous combustibility of Silane; a fire of a semiconductor factory in Kiyotake-cho, Miyazaki, caused by Silane gas leakage in October 1983, Silane gas explosion at a semiconductor prototyping facility in Kodaira, Tokyo, in December 1989, a fire caused by Silane at a semiconductor factory of Miyazaki Oki Electric in Takasaki, Gunma, in March 1990, a fire caused by Silane leakage at a Silane manufacturing plant in Aomi, Niigata, in June 1990. |
| Incidental Discussion |
Article 23 of the Constitution guarantees the freedom of learning as "Academic freedom shall be guaranteed." Some understand this constitution not only guarantees the academic freedom but also guarantees autonomy in universities. However, people should fully understand that university laboratories are not extraterritorial and should take every possible measure to ensure their safety. |
| Scenario |
| Primary Scenario
|
Unknown Cause, Occurrence of Abnormal Phenomenon, Usage, Operation/Use, Non-Regular Operation, Change in Operation, Bodily Harm, Death
|
|
| Sources |
[1] Contamination issues caused by chemical material, Hokkaido University information initiative Center. http://www.hucc.hokudai.ac.jp/
[2] Machinery Creativity, Yotaro Hatamura, Kozo Ono, Masayuki Nakao (Editors), Maruzen Co., Ltd.
[3] The Practice of Machine Design Book 3, Learning from Failure, Yotaro Hatamura ed., Practice of Machine Design Research Group, The Nikken Kogyo Shimbun, Ltd.
[4] High-pressure Gas Vol.29 No.251 (1992), Osaka University Silane Gas Explosion Accident Investigative Committee Interim Report
|
| Number of Deaths |
2 |
| Number of Injuries |
5 |
| Multimedia Files |
Figure1.Piping outline around the containers for CVD system (estimation)[3]
|
|
Figure2.Check valve
|
|
Figure3.Piping layout of gas supply facility
|
| Field |
Mechanical Engineering
|
| Author |
NAKAO, Masayuki (Institute of Engineering Innovation, School of Engineering, The University of Tokyo)
|
|