| Case Name |
Explosion due to flame during welding to install piping to a neutralization tank at a hydrocarbon resin plant |
| Pictograph |
|
| Date |
November 2, 1983 |
| Place |
Kurashiki, Okayama, Japan |
| Location |
Chemical factory |
| Overview |
The coagulating sedimentation tank was installed in the waste water treatment facilities. Neutralization and aeration were carried out with injecting a small amount of sulfuric acid, while waste water was circulating. Hot work to install waste water return piping started while operation of the tank continued. The explosion occurred soon after welding for temporary tacking was carried out in the tank. C5 fractions, which existed in small quantity in the waste water due to aeration evaporated, and became a combustible gas-air mixture. It was ignited by welding sparks. |
| Incident |
The waste water treatment facilities were being operated for the first time after the coagulating sedimentation tank had been added. As hot work to install the tank still continued, flammable gas generated during the operation ignited by welding sparks and exploded. |
| Processing |
Manufacture |
| Individual Process |
Waste water and waste oil treating |
| Process Flow |
Fig2.Unit process flow
|
| Substance |
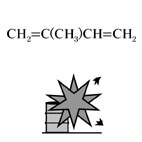
Isoprene, Fig3 |
| Type of Accident |
Explosion |
| Sequence |
Between October 25th and 31st, 1983, a coagulating sedimentation tank was installed in waste water treatment facilities.
10:30, on November 2nd; neutralization and aeration started with injecting a small amount of sulfuric acid, while waste water was circulated in the neutralization tank.
13:00: work to install a return line of waste water from the tank started while operation of the tank continued.
16:23; an explosion occurred soon after welding for temporary tacking was carried out in the tank. |
| Cause |
As a result of aeration, C5 fractions which existed in small quantity in waste water evaporated, and became a combustible gas mixture. It was ignited by welding sparks and exploded. |
| Countermeasures |
1. Hot work must not be allowed during operation. The safety checks before work are obligatory.
2. The facilities and operation were modified to control the generation of combustible gas.
3. Measures such as introducing the nitrogen gas blanket system into the neutralization tank are carried out to control the evaporation or vaporization of combustible gas. |
| Knowledge Comment |
It is important to recognize the danger of carrying out operation and maintenance at one time. The fact that waste water and its treatment are dangerous should be recognized. |
| Background |
Ignorance of the danger of waste water processed by an oil separator was the main factor of the accident. It is important to recognize that waste water is a hazardous material because it dissolves materials that have contacted in the process. The biggest problem is that hot work restarted while the waste water treatment facilities of the C5 hydrocarbon resin System was in operation. |
| Incidental Discussion |
There was a mistaken belief that treating waste water processed by an oil separator has no danger of fire. |
| Reason for Adding to DB |
A typical accident caused due to failure to predict the generation of hazardous materials |
| Scenario |
| Primary Scenario
|
Misjudgment, Misperception, Mis-Convincement, Poor Value Perception, Poor Safety Awareness, Insufficient Safety Measure, Organizational Problems, Inflexible Management Structure, Insufficient Education/Training, Planning and Design, Poor Planning, Pepair Work Schedule Planning, Usage, Maintenance/Repair, Welding, Malicious Act, Rule Violation, Safety Rule Violation, Secondary Damage, External Damage, Explosion, Bodily Harm, Death, 1person died, Bodily Harm, Injury, 3 person injured, Loss to Organization, Economic Loss, Tank Failure
|
|
| Sources |
High Pressure Gas Safety Inst. of Japan. Hydrocarbon resin plant. Explosion during piping mounting work at a waste water neutralization tank caused by welding sparks. Accident examples of Petroleum refinery and Petrochemical unit. pp.149-151(1995)
|
| Number of Deaths |
1 |
| Number of Injuries |
3 |
| Multimedia Files |
Fig3.Chemical formula
|
| Field |
Chemicals and Plants
|
| Author |
WAKAKURA, Masahide (Kanagawa Industrial Technology Research Institute)
TAMURA, Masamitsu (Center for Risk Management and Safety Sciences, Yokohama National University)
|