| Case Name |
Explosion due to hypergolic hazards in the intermediate tank of a propylene oxide manufacturing plant |
| Pictograph |
|
| Date |
June 11, 1964 |
| Place |
Kawasaki, Kanagawa, Japan |
| Location |
Chemical factory |
| Overview |
On June 11th, 1964: An explosion occurred when liquid in the distillation column was transported to the crude PO intermediate tank during repairs of a propylene oxide manufacturing plant. The alkali in the transfer liquid caused an exothermic reaction with propylene oxide (PO). A lack of consideration to hazards and properties of the material seems to have been a problem. |
| Incident |
At a propylene oxide (PO) manufacturing plant, residual liquid in the rectifying column was transferred into a crude PO intermediate tank for repairs of the column. Just after the transfer finished, white smoke rose near the tank, a large explosion occurred, and a fire broke out. There were two series of PO manufacturing plants. Refer to Fig2. |
| Processing |
Manufacture |
| Individual Process |
Other |
| Process Flow |
Fig3.Unit process flow
|
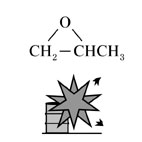
| Substance |
Propylene oxide, Fig4 |
| Type of Accident |
Burst, leakage, Fire, explosion |
| Sequence |
14:10 on June 11th, 1964: Transfer of bottom liquid from the #1 rectifying column of the #1 plant to crude PO intermediate tanks A,B (SS41, about 14 cubic meters) of the #2 plant started in preparation for repair of the column in the #1 plant.
14:50: Transfer finished after about 1 hour.
About 15:50: The alarm at the top of the #2 reactor of the #2 plant sounded. The overhead pressure rose above 0.03 MPaG, increasing of flow rate to the #1 rectifying column of the #2 plant was observed, and temperature of the column bottom started drifting.
About 15:06: White smoke rose around the intermediate tank A.
15:07. A strong explosion occurred in the intermediate tank A. |
| Cause |
The composition of the bottom liquid of the #1 rectifying column contained alkaline water, isopropanol, etc., and the temperature was around 85 °C due to the steam leak. Originally, the temperature of the intermediate tank should have been kept at 20 °C or less. The hot bottom liquid contacted PO in the tank, and there was an exothermic polymerization reaction of propylene oxide with an alkali catalyst started, and eventually a runaway reaction continued. As a result, the pressure rose, and the tank ruptured. As the tank's internal pressure fell rapidly, the residual liquid in the tank instantaneously evaporated (vapor explosion). Continuously PO vapor, etc, that spouted into the air caused the vapor cloud explosion.
Vapor cloud explosion: An explosion of combustible gas that dispersed in the atmosphere like a cloud in the sky. It brings extensive damage. |
| Countermeasures |
The crude PO intermediate tank and the blowdown tank are separated. |
| Knowledge Comment |
A blowdown tank might have to accept various compositions or materials with various properties. Therefore, discussion on the potential hazards of incompatibility even in an emergency should be considered from chemical and chemical-engineering points of view when the intermediate tank for crude products is also used as a blowdown tank. |
| Background |
The first problem may be giving the role of a blowdown tank of the alkaline solution in an emergency to the intermediate tank for crude propylene oxide. It did not seem that the contamination reaction hazard of PO and alkali was taken into account at the process design stage. About operation management, the tank temperature, which was regulated, was not kept. Although the effects were not clarified, there is no doubt that high temperature accelerated the reaction rate. It is also a problem that the safety countermeasures in the design were insufficient. Countermeasures might have been taken if the operating personnel had checked the contamination reaction hazard of PO and alkali as it is very difficult to re-evaluate the process design. In conclusion, the accident was caused by neglecting a material characteristics investigation in the design, and errors in operation management.
As PO is a liquid with a boiling point of 34 °C, and vapor pressures of 2.5 MPaG at 170 °C and 3 MPaG at 180 °C, it is necessary to have a temperature of 170-180 °C to reach the burst pressure of the tank. PO causes anionic polymerization with an exothermic reaction in the presence of alkali. Heat of polymerization is about 18 kcal/mol, and 180 °C can be reached with even partial polymerization of the liquid. |
| Incidental Discussion |
Now, it is common sense for a person involved in PO plant operation to know that PO will polymerize in the presence of alkaline. We should more investigate the risk and a sense of danger and experience are required. |
| Reason for Adding to DB |
Example of accident caused due to lack of basic knowledge on material hazards |
| Scenario |
| Primary Scenario
|
Poor Value Perception, Poor Safety Awareness, Insufficient Safety Measure, Ignorance, Insufficient Knowledge, Insufficient Study/Experience, Planning and Design, Poor Planning, Poor Design, Regular Operation, Erroneous Operation, Temperature abnormal high, Bad Event, Chemical Phenomenon, Abnormal Reaction, Secondary Damage, External Damage, Explosion, Bodily Harm, Death, 8 person died, Bodily Harm, Injury, 117 person injured, Loss to Organization, Economic Loss, Tank Completely Raptured
|
|
| Sources |
Tetsuzo Kitagawa, Explosion caused by runaway reaction of propylene oxide intermediate tank. Analysis of explosion hazard. pp.235-249(1980)
High pressure gas Inst. Of Japan, Accident examples in Complexes, pp.205-208(1991)
Japan property insurance association, Cases of disaster. Oil tank, Fire prevention guideline 12. Fire prevention and explosion-proof guideline for tanks. pp.112-113(1970)
Chemical Industries Association, Cases of accidents with tanks, Cases of accidents and countermeasures. 4, safety countermeasure technologies for chemical plants. pp.285-286(1979).
|
| Number of Deaths |
18 |
| Number of Injuries |
117 |
| Physical Damage |
A tank completely collapsed, adjacent buildings were destroyed by fire. |
| Multimedia Files |
Fig2.Field photograph
|
|
Fig4.Chemical formula
|
| Field |
Chemicals and Plants
|
| Author |
ARAI, Mitsuru (Environmental Science Center, The University of Tokyo)
TAMURA, Masamitsu (Center for Risk Management and Safety Sciences, Yokohama National University)
|
|