| Case Name |
Explosion of acrylic acid under storage in a drum can after partial melting |
| Pictograph |
|
| Date |
January 19, 1969 |
| Place |
Yokohama, Kanagawa, Japan |
| Location |
Chemical factory |
| Overview |
An explosion occurred on the first floor of a factory at a stainless steel drum can of 200 L capacity holding acrylic acid. The lid of the drum can was blown off and a gas explosion occurred with the ignition to the spouting gas in the air. The four-floor factory building collapsed. Partial melting of acrylic acid that had solidified was done in order to take out a part of it many times. The polymerization inhibitor transferred to a liquid state during repeated melting and solidifying, and the concentration in the solidified state gradually dropped. |
| Incident |
Five drum cans of acrylic acid, which were received about one month before, solidified because of low temperature. For using the acid, one out of five drum cans was melted using 100 V, 750 W band heaters, and the melted acid was taken out using a plastic hand pump. The work was repeated many times. The can exploded on the third day after the heater was turned off and the can was capped. The lid of the drum can blew off and there was a vapor explosion of about 100 L of acrylic acid because of a rapid pressure drop. The mist dispersed in the air ignited and exploded |
| Processing |
Manufacture |
| Individual Process |
Dissolution |
| Substance |
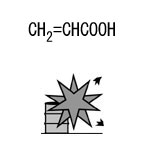
Acrylic acid, Fig2 |
| Type of Accident |
Leakage, Fire |
| Sequence |
Solidified acrylic acid in the drum can was partially melted by heating. Heating was done using 750 W band heaters, which wrapped around the side of the can, and glass fiber covered all surface of the drum. The heater was turned on and the melted acid was pumped out. This work was repeated three times. The explosion occurred three days after the last work. First, a spontaneous polymerization reaction occurred, and the temperature rose due to the reaction heat, and a runaway reaction occurred. Vapor pressure rose, and the lid of the drum can blew off. Due to a rapid pressure drop, there was a vapor explosion of about 100 L of acrylic acid that remained in the drum can. The mist dispersed in the air ignited and exploded. |
| Cause |
The following cycle was repeated: Partial melting, taking out of liquid, solidification. The polymerization inhibitor for preventing a runaway reaction was transferred from solidified acid to liquid acid. As a result, the concentration of the inhibitor in the solidified acrylic acid seems to have become lower. With contamination by iron rust during the work or in the austenitic stainless steel material of the drum can, a polymerization reaction was promoted, and a runaway reaction progressed. |
| Countermeasures |
Taking out liquid acid after it has all completely melted. |
| Knowledge Comment |
1. Freezing and melting points of base materials and additives are usually different. Therefore, repeated cycles of partial melting and solidification changes the concentration of the additives.
2. If there is a polymerization inhibitor in the acid, it might be removed under various conditions, and lose its effectiveness. |
| Background |
The polymerization inhibitor melts easier than acrylic acid when it is heated, thus the inhibitor transfers from solidified acrylic acid to liquid acrylic acid. However, this might not have been understood at that time. An accident was inevitable. |
| Reason for Adding to DB |
Example of explosion caused due to decreasing polymerization inhibitor concentration through careless work |
| Scenario |
| Primary Scenario
|
Insufficient Analysis or Research, Insufficient Practice, Lack of Imagination, Poor Value Perception, Poor Safety Awareness, Insufficient Recognition of Risk, Ignorance, Insufficient Knowledge, Insufficient Experience, Planning and Design, Poor Planning, Poor Design, Usage, Operation/Use, Dissolution Method, Bad Event, Chemical Phenomenon, Concentration Change, Secondary Damage, External Damage, Explosion, Loss to Organization, Economic Loss, Factory Building Completely Destructed
|
|
| Sources |
Masamitsu Tamura, Masahide Wakakura, Acrylic acid Explosion. Reaction danger,-Accident case and analysis- p.146(1995)
Tetsuzo Kitagawa, Explosion caused by runaway polymerization of acrylic acid container. Analysis of explosion hazard, p.250(1980)
|
| Physical Damage |
A steel-frame, four-floor factory building with a slate roof collapsed. |
| Multimedia Files |
Fig2.Chemical formula
|
| Field |
Chemicals and Plants
|
| Author |
ARAI, Mitsuru (Environmental Science Center, The University of Tokyo)
TAMURA, Masamitsu (Center for Risk Management and Safety Sciences, Yokohama National University)
|
|