| Case Name |
Explosion in a storage tank caused due to decomposition of a polymerization catalyst |
| Pictograph |
|
| Date |
December 29, 1995 |
| Place |
Yokkaichi, Mie, Japan |
| Location |
Chemical factory |
| Overview |
An organic peroxide catalyst exploded in a polystyrene plant. The problem of catalyst solidifying in piping from a tank to a reactor was repeated due to a temperature fall. For this reason, steam piping was rolled and heated. Piping of a spare pump, which was stopped for repair, was also heated. Therefore, the catalyst at the side of the spare pump reached a high temperature over a self-exothermic decomposition temperature, and began to decompose. The catalyst tank was heated by a reverse flow of the high temperature gas, which was generated during decomposition, and the tank exploded. The heat properties of the catalyst were not fully grasped. |
| Incident |
An explosion of organic peroxide used as a polymerization catalyst occurred at a polystyrene plant. A steam trace was carried out with catalyst piping in December when the temperature fell. At that time, in a storage tank for organic peroxide used as a catalyst, self-exothermic decomposition was caused and then an explosion occurred. Surrounding buildings and apparatus were damaged.
An organic peroxide catalyst was t-butyl peroxybenzoate. |
| Processing |
Manufacture |
| Individual Process |
Charge and Feed |
| Chemical Reaction |
Polymerization |
| Substance |
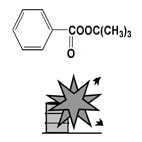
t-Butyl peroxybenzoate, Fig2 |
| Styrene, Fig3 |
| Type of Accident |
Explosion |
| Sequence |
A catalyst solidified in feed piping to the reactor during operation of a polystyrene plant due to a fall of temperature. The feed rate of the catalyst decreased. For this reason, the catalyst was melted by pouring warm water on pumps, filters, and piping with hoses.
The problem was repeated over several days and dealt with each time.
It was decided to improve the facilities by rolling steam piping around the feed piping for heating.
On December 2nd, 1995. Steam pipe rolling was executed from the pumps outlet to the flow meter.
On December 27th. Furthermore, steam pipe rolling was executed from the tank to the pumps inlet.
About 17:00. Steam was fed and heating started.
About 21:50 on December 29th. Freezing occurred again and steam was applied with a hose to piping. Solidified material was melted. The hose was left as it was and steam continued to be supplied.
About 22:00. Trouble with a low flow rate occurred again. A strainer was overhauled after stopping steam supply, but the problem was not recovered.
About 22:30. The catalyst tank was checked. As a pressure gauge had swung past the maximum and was out of order, it was replaced. Just before the explosion, when a drain valve of the catalyst feed line was opened, a lot of gas blew off.
23:01. Operators sensed a hazard, and began to take refuge in the control room. An explosion occurred, before complete evacuation to the control room.
23:03. The fire station was notified about the accident. Three fire engines and 15 fire fighters turned out. Water was sprayed on equipment for cooling by public and private fire fighters.
11:40 on December 30th. Treatment was completed. |
| Cause |
Piping from the tank to the reactor was heated by steam to prevent solidification. There were two feed pumps including a spare. Piping for the spare pump was also heated. As the temperature of the piping for the spare pump exceeded the self-exothermic decomposition temperature of the catalyst, it began to decompose. Furthermore, hot decomposition gas flowed backwards into the tank, the temperature in the tank rose, and the catalyst in the tank decomposed and exploded. |
| Response |
Two fire engines and ten self-defense fire fighters, three fire engines and 15 public fire fighters turned out. Water was sprayed on equipment for cooling. |
| Countermeasures |
Organic peroxide is used in a diluted condition by a solvent to prevent solidification.
A temperature indicator and a temperature alarm are installed in the catalyst tank.
Dead-end spaces of feed piping are minimized.
Work standards for unusual work are reviewed.
Safety education and training are strengthened. |
| Knowledge Comment |
Material used as a catalyst or a reaction initiator often has high reactivity, and is thermally unstable.
The concentration of a catalyst in the reactor is not high. However, as the concentration in the catalyst tank and its piping is very high, it is necessary to understand the hazards of the material in advance.
Piping of the spare pump is dead-end piping, and there is no flow. As various behaviors in dead-end piping differ from those in the place where there is a flow, sufficient caution is required. |
| Background |
By heating a dead-end space, the temperature rose over a self-exothermic decomposition starting temperature.
Self-exothermic decomposition and thermal stability of an organic peroxide catalyst were not fully understood.
When a dead-end space was heated, it was not known that the temperature rose to the almost same level as a heating medium. The accident was caused by a lack of safety knowledge. |
| Incidental Discussion |
This accident also resulted from a dead-end space. Sufficient consideration is needed when heating a no-flow place. |
| Reason for Adding to DB |
Example of explosion caused due to an unexpected temperature rise from heating a place with no flow |
| Scenario |
| Primary Scenario
|
Ignorance, Insufficient Knowledge, Insufficient Study, Poor Value Perception, Poor Safety Awareness, Inadequate Risk Recognition, Organizational Problems, Poor Management, Poor Operation Management, Planning and Design, Poor Planning, Poor Heating Planning, Non-Regular Action, Change, Change of Heating Method, Bad Event, Chemical Phenomenon, Abnormal Reaction, Secondary Damage, External Damage, Explosion, Bodily Harm, Death, Loss to Organization, Economic Loss, Manetary Damage 50 million yen
|
|
| Sources |
Fire and Disaster Management Agency, Explosion caused by decomposition reaction of catalyst. Accident cases of dangerous materials. 1995. pp.340-341.
|
| Number of Deaths |
1 |
| Physical Damage |
Damage to 6.6 square meters of apparatus on the first floor, about 120.4 square meters of the second floor, about 45.4 square meters of the third floor, and about 12.5 square meters of the fourth floor. Damage to windows of 18 surrounding buildings, and slates blew off. |
| Financial Cost |
¥ 50 million (Fire and Disaster Management Agency) |
| Multimedia Files |
Fig2.Chemical formula
|
|
Fig3.Chemical formula
|
| Field |
Chemicals and Plants
|
| Author |
ITAGAKI, Haruhiko (Japan National Institute of Occupational Safety and Health)
TAMURA, Masamitsu (Center for Risk Management and Safety Sciences, Yokohama National University)
|
|